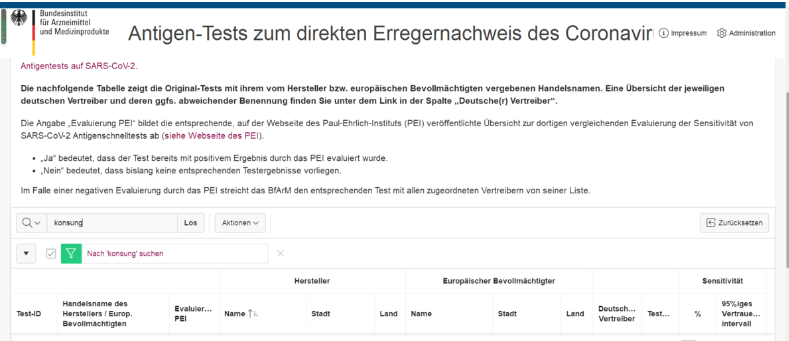
February 24th, COVID-19 antigen test kit and COVID-19 salivary antigen test kit independently developed by Konsung Medical have successfully obtained the notice of admission from Federal Institute for Drugs and Medical Devices (BfArM) Germany. It helps official German institutions carry out large-scale rapid screening of the new coronavirus, to ensure public welfare, assist safe returning to work and school.
Jens Spahn, head of Federal Health Ministry of Germany, told the media in an interview that, from March 1st, Germany will be capable of providing more novel coronavirus rapid test, and all the citizens can get virus detection for free.
He also revealed the fact that, concerned administrations were speeding up examination on unprofessional institutions, to offer permission for their authority of providing rapid antigen detection. By the local time of evening of February 22nd, the population of new coronavirus infection in Germany had exceeded to more than 2.4 million. Germany has the market of tens of millions of people, which brings huge demand. And the market of home self-testing will be open soon.
BfArM(Bundesinstitut für Arzneimittel und Medizinprodukte)Federal Institute for Drugs and Medical Devices (BfArM) of Germany, responsible for the supervision of all chemicals, plant drugs and adjuvant drugs. As the subordinate body of the German Ministry of Health, it is responsible for market permission and drug alert of human use medicine.
COVID-19 salivary antigen test kit, a kind of salivary test product which has rarely been permitted in BfArM, Germany. The product has no need to use specific swabs. The sample collection way of collecting saliva into a sterile chamber can release uncomfortableness of patients. The accuracy of these two products is high. They can be used in group rapid screening and assisting rapid diagnosis of suspected patients. And the suspected infected patients can be isolated in time, which can meet the need of rapid screening on the spot in epidemic prevention and control of all countries.
|
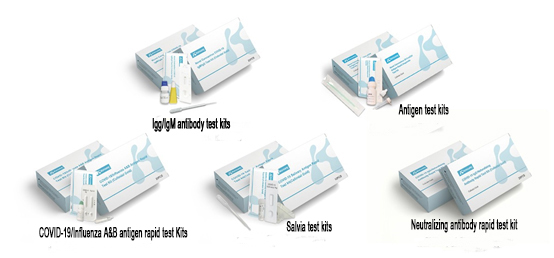
Product Name |
Sampling Method |
Main use |
|
COVID-19 Antigen Rapid Test Kit (Colloidal Gold) |
Nasal swab; throat swab |
Early screening and diagnosis, is applied for primary large-scale rapid screening |
|
COVID-19 Salivary Antigen Rapid Test Kit (Colloidal Gold) |
Saliva |
|
|
Novel Coronavirus COVID-19 IgM/IgG Test Kit (Colloidal Gold) |
fingertip |
Is applied for large-scale tests of suspected cases and asymptomatic patients |
|
COVID-19 Neutralizing Antibody Rapid Test Kit (Colloidal Gold) |
fingertip |
Is applied for COVID-19 vaccine evaluation |
|
COVID-19/Influenza A&B Antigen Rapid Test Kit (Colloidal Gold) |
Nasal swab; throat swab |
Is applied for detection in epidemic frequency period, for differentiation of Flu and COVID-19 infection, help the diagnosis and treatment |
Besides, Konsung Medical is working on enriching the variety of novel coronavirus detection products. At present, Novel Coronavirus COVID-19 IgM/IgG Test Kit, COVID-19 Neutralizing Antibody Rapid Test Kit, COVID-19/Influenza A&B Antigen Rapid Test Kit are all during registration. Konsung Medical is forging rapid test resolution of COVID-19 test kits, which can meet various of scene and clinical demands.
Nowadays, COVID-19 is still in the important stage of prevention and control, variable factors will put forwarder highly requirements continuously for the measure of prevention and control, detection and diagnostic. Konusng medical will overcome technique difficulty, shoulders more community responsibilities, and contribute to global anti epidemic.
Post time: Feb-26-2021