April 8th, 2021, COVID-19/Influenza A & B-Antigen test kits independently developed by Konsung medical have successfully obtained the announcement of admission from Federal Institute for Drugs and Medical Devices (BfArm), Germany. After the antigen and saliva test kits, it is the third kind of rapid test kits for COVID-19

which obtained the market license from German BfArM. This rapid test kits will help official German institutions carry out large-scale rapid screening of the SARS-CoV-2, to ensure public welfare.
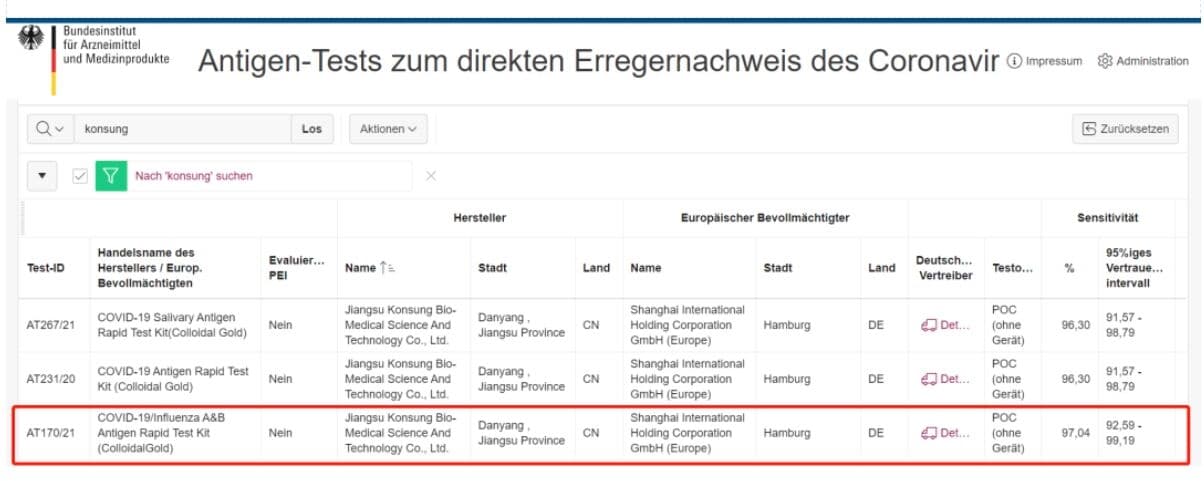
BfArM(Bundesinstitut für Arzneimittel und Medizinprodukte) is the Federal Institute for Drugs and Medical Devices, which supervises all chemicals, plant medicines and adjuvant.
Influenza (Flu) and COVID-19 are both contagious respiratory diseases, which can be spread via contact, droplets, etc. The symptoms of the novel corona virus and
influenza virus are similar. Both illnesses can cause fever, cough, body ache, and may result in pneumonia or severe lung infection. Konsung COVID-19/Influenza A&B Antigen Rapid Test Kit (Colloidal Gold) can realize multiplex tests to differentiate the cause of symptoms between COVID-19 and flu with single one throat or nasal swab sample to reduce the burden of healthcare system and meet the requirements of on-site rapid screening in different countries.
Post time: Apr-09-2021
